FREEDOM Study
The FREEDOM (Fracture REduction Evaluation of Denosumab in Osteoporosis Every 6 Months) study is Prolia’s pivotal phase III, randomized, double-blind, controlled study completed with 7,808 postmenopausal women. 1,2
The safety profile and efficacy of Prolia were evaluated in the FREEDOM trial and the results were published in the New England Journal of Medicine. FREEDOM’s primary endpoint was the incidence of new vertebral fracture over a period of 36 months. Secondary endpoints included the time to first nonvertebral fracture and time to first hip fracture.2
7,808 women between the ages of 60 and 91 years old with BMD T scores less than -2.5 and not less than -4.0 at the lumbar spine or total hip were enrolled in FREEDOM and randomized to receive either Prolia or placebo.1*
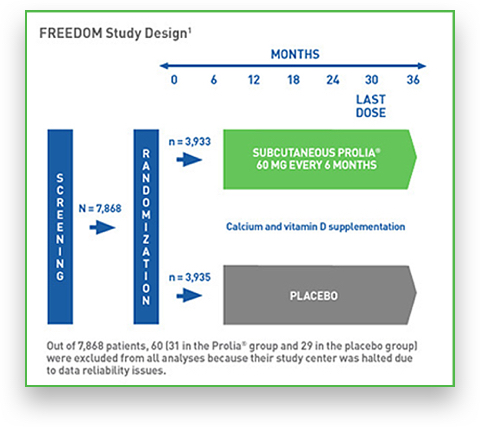
* Of these subjects, 60 (31 in the Prolia group and 29 in the placebo group) were excluded from all analyses because the participation of their study centre was halted owing to issues related to study procedures and the reliability of data.2
Key Findings
The key finding in the 3-year study was that patients taking Prolia had a significant reduction in the risk of new fracture at all measured sites (vertebral, hip, nonvertebral) compared with those taking placebo.
In the long-term, open-label extension, among 2,343 women who received Prolia in FREEDOM and continued on therapy (years 4 through 10 of Prolia treatment), 1,343 (57.3%) completed 10 years. Prolia treatment maintained a low incidence of new vertebral fractures in years 4 through 10 (7.0% had at least one new vertebral fracture).1
New vertebral fractures
Prolia
placebo

Hip fractures
Prolia
placebo

Non-vertebral fractures
Prolia
placebo

In the long-term, open-label extension, Prolia treatment also continued to increase BMD from extension baseline at the lumbar spine (10.8%; n=1,264), total hip (3.4%; n=1,232), and femoral neck (3.8%; n=1,232) over years 4 through 10. Percent increase in BMD from the original FREEDOM study baseline (i.e. after 10 years of treatment) in the long-term group was 21.7% at the lumbar spine, 9.2% at the total hip, and 9.0% at the femoral neck.
ARR=absolute risk reduction; BMD=bone mineral density; FRR=fracture risk reduction; RRR=relative risk reduction
* Annualized yearly subject incidence.
† In women who received Prolia in the 3-year, placebo-controlled phase
and continued on therapy in the long-term, open-label extension.
Additional Resources
References
| 1. | Amgen Canada Inc. Prolia® (denosumab injection) Product Monograph. November 24, 2023. |
| 2. | Cummings SR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756-765. |


